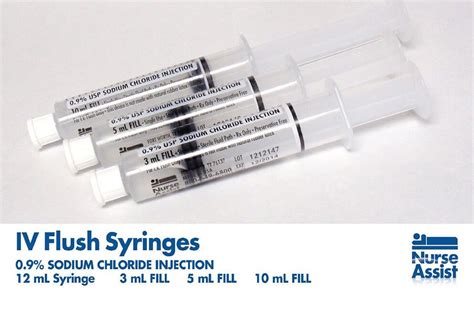
saline flush lv bag | saline flush syringe recall saline flush lv bag The plastic syringes contain normal saline (0.9 percent sodium chloride). In . 50: DRK Req. Level 50 Physical Damage 51 57 Magic Damage 26 29 Strength +12 +13 Vitality +15 +17 Skill Speed +22 +24 Determination +15 +17 Senor Sabotender's Spiked RodDark Knight - Part 1: 1 Dark Knight's Armor Coffer (IL 90) The Wages of Mercy: 50 Unflinching Temple Knight: The Knight and the Maiden Fair: 52 Sidurgu Salted Earth: Kindred Spirits: 54 Sidurgu Plunge: Original Sins: 56 Sidurgu Abyssal Drain: The Flame in the Abyss: 58 Sidurgu: Absolution: 60 Sidurgu Carve and Spit A Dark Day's .
0 · saline needle insert safety
1 · saline flush vs prefilled
2 · saline flush syringe recall
3 · pre filled syringe for saline
4 · pre filled saline flush
5 · iv flushing syringe prefilled
6 · iv flush syringe safety
7 · iv flush administration uk
Primal Glamour Weapons (lvl 60) 180: Crafted, level 60★★ & 60★★★ recipes (Master Recipes IV) Doman Weapons (Kai) 180: Purchased from Yolaine in Foundation (X:13.0 Y:11.8) for 70 Centurio Seals: Hive Weapons: 190: Drops in Thok ast Thok (Extreme) or trade 10 Hive Totem to Bertana in Idyllshire.
The plastic syringes contain normal saline (0.9 percent sodium chloride). In . The FDA reclassified all forms of pre-filled heparin and pre-filled saline flushes . The plastic syringes contain normal saline (0.9 percent sodium chloride). In March 2022, the FDA announced a shortage of prefilled 0.9% sodium chloride IV flush syringes because of increased demand, vendor supply chain challenges and discontinuation of particular flushes. In the UK, the Medicines and Healthcare products Regulatory Agency classifies ‘pre-filled syringes’ for flushing Intravenous (IV) cannulas and IV access devices as ‘borderline’ devices and offers some advice on how control measures can help mitigate risks.
The FDA reclassified all forms of pre-filled heparin and pre-filled saline flushes as medical devices. Previously, they were classified as either a device or a drug depending on how the manufacturer submitted its application to the FDA. Health care professionals routinely use saline flush syringes to keep patients’ IV lines air-free and unobstructed. When manufactured and used correctly, the devices are generally safe, but problems can occur.
We carry a full range of saline bags including several options of normal saline bags as well as other saline solutions. We also offer prefilled syringes and specialized IV bags such as lactated ringers, dextrose, heparin, multiple electrolyte solutions, and more. Another issue is dilution or reconstitution of I.V. push medication using a prefilled 0.9% sodium chloride (saline) flush syringe. Nurses are making more work for themselves by diluting drugs unnecessarily, and the practice doesn’t provide value to patients; instead, it creates contamination and medication error risks.

Line flushing is needed to prevent medi-cine loss after IV fluid therapy but is rarely standard practice. Post-infusion flushing is required for small-volume infusions to ensure patient safety and therapy effective - ness. Accidental push dosing can occur if the correct flushing technique is not used. NT This article is a summary of NIVAS’sPurchase Saline Solution IV Bags for sale at competitive prices. Browse our medical infusion and IV bag catalog. Order Online or Call Now! The concept of one and done also applies to the use of prefilled flush syringes (PFS), which have been available since 1999 and are used each time an I.V. catheter is accessed for maintenance flushes or to administer medications.
The only prefilled saline flush syringe with an attached disinfection cap soaked in 70% isopropyl alcohol. Flush and protect lines in 2 simple steps. The plastic syringes contain normal saline (0.9 percent sodium chloride). In March 2022, the FDA announced a shortage of prefilled 0.9% sodium chloride IV flush syringes because of increased demand, vendor supply chain challenges and discontinuation of particular flushes. In the UK, the Medicines and Healthcare products Regulatory Agency classifies ‘pre-filled syringes’ for flushing Intravenous (IV) cannulas and IV access devices as ‘borderline’ devices and offers some advice on how control measures can help mitigate risks.
The FDA reclassified all forms of pre-filled heparin and pre-filled saline flushes as medical devices. Previously, they were classified as either a device or a drug depending on how the manufacturer submitted its application to the FDA. Health care professionals routinely use saline flush syringes to keep patients’ IV lines air-free and unobstructed. When manufactured and used correctly, the devices are generally safe, but problems can occur.

We carry a full range of saline bags including several options of normal saline bags as well as other saline solutions. We also offer prefilled syringes and specialized IV bags such as lactated ringers, dextrose, heparin, multiple electrolyte solutions, and more.
Another issue is dilution or reconstitution of I.V. push medication using a prefilled 0.9% sodium chloride (saline) flush syringe. Nurses are making more work for themselves by diluting drugs unnecessarily, and the practice doesn’t provide value to patients; instead, it creates contamination and medication error risks.Line flushing is needed to prevent medi-cine loss after IV fluid therapy but is rarely standard practice. Post-infusion flushing is required for small-volume infusions to ensure patient safety and therapy effective - ness. Accidental push dosing can occur if the correct flushing technique is not used. NT This article is a summary of NIVAS’sPurchase Saline Solution IV Bags for sale at competitive prices. Browse our medical infusion and IV bag catalog. Order Online or Call Now!
saline needle insert safety
The concept of one and done also applies to the use of prefilled flush syringes (PFS), which have been available since 1999 and are used each time an I.V. catheter is accessed for maintenance flushes or to administer medications.
saline flush vs prefilled
1. Overview. White Mage starts the game as Conjurer at level 1 and becomes White Mage at level 30 upon doing the job quest "Seer Folly" at level 30. This guide will start at level 15, the lowest level you can be synced to by a dungeon.Level IL Obtained Weathered Crafting and Gathering Tools: 1: 1: Lv. 1 class quest reward; purchased from NPC vendors. Level 1-10 Crafting and Gathering Tools: 1-10: Crafted, quest rewards Level 11-20 Crafting and Gathering Tools: 11-20: Crafted, quest rewards Level 21-30 Crafting and Gathering Tools: 21-30: Crafted, quest rewards
saline flush lv bag|saline flush syringe recall